UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 OR 15(d) of The Securities Exchange Act of 1934
Date of Report (date of earliest event reported): January 12, 2023 (January 12, 2023 )
(Exact name of registrant as specified in its charter)
(State or other jurisdiction of incorporation or organization) | (Commission File Number) | (I.R.S. Employer Identification No.) | ||||||||||||||||||
(Address of Principal Executive Offices) | (Zip Code) | |||||||||||||||||||
(800 ) 298-6470
Registrant's telephone number, including area code
(Former name or former address, if changed since last report.)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered | ||||||||||||
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.☐
Item 7.01 Regulation FD Disclosure.
On January 12, 2023, GeneDx Holdings Corp. (the "Company") is furnishing as Exhibit 99.1 hereto a copy of the investor presentation to be used at the 41st Annual J.P. Morgan Healthcare Conference event.
The information in this current report on Form 8-K and the exhibit attached hereto shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”) or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, regardless of any general incorporation language in such filing.
Item 9.01 Financial Statements and Exhibits
(d) Exhibits
Exhibit No. | Description | |||||||
99.1 | ||||||||
104 | Cover Page Interactive Data File (embedded within the Inline XBRL document) | |||||||
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| GeneDx Holdings Corp. | |||||||||||
| Date: | January 12, 2023 | By: | /s/ Katherine Stueland | ||||||||
| Name: | Katherine Stueland | ||||||||||
| Title: | Chief Executive Officer | ||||||||||

41st Annual JPMorgan Healthcare Conference January 12, 2023 San Francisco One Test: Miss Less. Discover More. GeneDx (Nasdaq: WGS) Katherine Stueland President and Chief Executive Officer

2 Disclaimer This presentation contains forward-looking statements under the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements are statements that do not relate to historical facts and events and such statements and opinions pertaining to the future that, for example, contain wording such as “may,” “might,” “will,” “could,” “would,” “should,” “expect,” “intend,” “plan,” “objective,” “anticipate,” “believe,” “estimate,” “predict,” “potential,” “continue,” “ongoing,” or the negative of these terms, or other comparable terminology intended to identify statements about the future. Forward-looking statements contained in this presentation include, but are not limited to, statements about: our future performance and our market opportunity, including our preliminary, unaudited pro forma revenue, pro forma test result volumes and pro forma adjusted gross margins for 2022, our expected full year 2023 reported revenue guidance, our expectations regarding our gross margin profile in 2023 and beyond, our use of cash for continuing operations and our cash burn in 2023 and our turning profitable in 2025, our expectations for our growth and future investment in our business, our expectations regarding our plans to pursue a new strategic direction and exit our reproductive health and somatic tumor testing businesses, including statements regarding our future performance and our market opportunity, including our preliminary, unaudited pro forma revenue, pro forma test result volumes and pro forma adjusted gross margins for 2022, our expected full year 2023 reported revenue guidance, our expectations regarding our gross margin profile in 2023 and beyond, our use of cash for continuing operations and our cash burn in 2023 and our turning profitable in 2025, our expectations for our growth and future investment in our business, our expectations regarding our plans to pursue a new strategic direction and exit our reproductive health and somatic tumor testing businesses,, our addressable market, market growth, future revenue, key performance indicators, expenses, capital requirements and our needs for additional financing, our commercial launch plans, our strategic plans for our business and products, market acceptance of our products, our competitive position and developments and projections relating to our competitors, domestic and foreign regulatory approvals, third-party manufacturers and suppliers, our intellectual property, the potential effects of government regulation and local, regional and national and international economic conditions and events affecting our business. We cannot assure that the forward-looking statements in this presentation will prove to be accurate. Furthermore, if our forward-looking statements prove to be inaccurate, the inaccuracy may be material. These statements involve known and unknown risks, uncertainties and other important factors that may cause our actual results, levels of activity, performance or achievements to be materially different from the information expressed or implied by these forward-looking statements. The forward-looking statements and opinions contained in this presentation are based on our management’s beliefs and assumptions and are based upon information currently available to our management as of the date of this presentation and, while we believe such information forms a reasonable basis for such statements, such information may be limited or incomplete, and our statements should not be read to indicate that we have conducted an exhaustive inquiry into, or review of, all potentially available relevant information. Many factors could cause actual future events to differ materially from the forward-looking statements in this presentation, including but not limited to: (i) the completion of the preparation of our 2022 year-end financial statements (including all required disclosures) and our 2022 year-end audit; (ii) the ability to implement business plans, goals and forecasts, and identify and realize additional opportunities, (iii) the risk of downturns and a changing regulatory landscape in the highly competitive healthcare industry, (iv) the size and growth of the market in which we operate, and (v) our ability to pursue our new strategic direction, and exit our reproductive health and somatic tumor testing businesses. The information, opinions and forward-looking statements contained in this announcement speak only as of its date and are subject to change without notice Use of Non-GAAP Financial Measures This presentation includes non-GAAP financial measures, including [Adjusted Gross Margin. We define Adjusted Gross Margin as our Adjusted Gross Profit divided by our revenue. Adjusted Gross Profit is a non-GAAP financial measure that we define as revenue less cost of services, excluding stock-based compensation expense and restructuring costs. Management believes that these non-GAAP measures of financial results are useful in evaluating the GeneDx's operating performance compared to that of other companies in its industry, as this metric generally eliminates the effects of certain items that may vary from company to company for reasons unrelated to overall operating performance. This presentation contains estimates, projections and other information concerning our industry, our business, and the markets for our products and services. Information that is based on estimates, forecasts, projections, market research or similar methodologies is inherently subject to uncertainties, and actual events or circumstances may differ materially from events and circumstances that are assumed in this information. Unless otherwise expressly stated, we obtained this industry, business, market and other data from our own internal estimates and research as well as from reports, research surveys, studies and similar data prepared by market research firms and other third parties, industry, medical and general publications, government data and similar sources. While we believe our internal company research as to such matters is reliable and the market definitions are appropriate, neither such research nor these definitions have been verified by any independent source. We discuss these and other risks and uncertainties in greater detail in the sections entitled “Risk Factors” and "Management's Discussion and Analysis of Financial Condition and Results of Operations" in our periodic reports and other filings we make with the SEC from time to time. Given these uncertainties, you should not place undue reliance on the forward-looking statements. Moreover, we operate in a very competitive and rapidly changing environment. New risks emerge from time to time. Except as required by law, we undertake no obligation to update publicly any forward-looking statements for any reason after the date of this presentation to conform these statements to actual results or to changes in our expectations. We file reports, proxy statements, and other information with the SEC. Such reports, proxy statements, and other information concerning us are available www.sec.gov. Requests for copies of such documents should be directed to our Investor Relations department at GeneDx Holdings Corp. 333 Ludlow Street, North Tower, 8th Floor, Stamford, Connecticut, 06902. Our telephone number is 800-298-6470.

Our mission: deliver personalized and actionable health insights to inform diagnosis, direct treatment and improve drug discovery.

The problem: lack of genomic data and connectivity with EMR Symptoms Tests are ordered More tests But no answers Try new doctors But no records Suboptimal care Waiting for answers that come too late Patients miss milestones Waiting for a proper diagnosis More tests And more spending Years of missed diagnosis leads to: • Inappropriate treatments • Increased costs on the healthcare system • Suffering for patients and families • Stress on providers • Missed opportunities to develop treatments and solutions

Our Solution: Genomic Data + Clinical Insights = Better Health

>400K clinical exomes > 1 million DNA extractions >400 gene discovery publications Built by GeneDx: Genomic interpretation platform Since our inception more than 20 years ago, we believe we offer the most definitive genomic analysis in the industry, borne from over a decade of constructing our proprietary data sets Invested in: • Scalable genome lab and informatics • New commercial strategy and team • Medical affairs and managed care >2.7 million phenotypes

Built by Sema4: Centrellis health insights platform We’ve spent over 10 years developing the most sophisticated dataset in the industry, complete with custom algorithms and more accurate analyses 3.1 million patient health records 8 million disease diagnoses 47 million phenotypes 20 years of records abstracted from 56 million clinical documents • Strengthening data asset for Centrellis • Reorganized product and technology team for scale • Discontinued reproductive health, somatic oncology, commercial operations

Best of both creates a seamless solution Sequence once Expand the use of and deliver best in class whole exome/genome analysis & interpretation Combine clinical + genomic data Match structured clinical and genomic data and supplement with disease models to drive insights Providing actionable insights - Leveraging clinical data adds a critical layer onto what we know from structured genomic data including, phenotypic information, symptoms, family history, and longitudinal data Centrellis® Generate genomic insights over time Generate patient-specific and disease profiles to generate actionable reports upon presentation of symptoms

9 The strategy is working: high volume and revenue growth, expanding gross margins, reduced cash burn Excluding revenues and costs from the exited business activities, we expect GeneDx to: • Generate pro forma revenues between $170-173 million in 2022, a 37-40% increase from 2021 • Produce pro forma test result volume of >180,000 in 2022, a 23% increase from 2021 • Deliver pro forma adjusted gross margin between 38-41% in 2022, a 34% increase from 20212 Preliminary 2022 Financial Results1 1. GeneDx has not completed the preparation of its financial statements for the year ended December 31, 2022. The preliminary, unaudited results presented in this presentation for the year ended December 31, 2022, are based on current expectations and are subject to adjustment, as the company completes the preparation of its 2022 year-end financial statements and its 2022 year-end audit. Further, the preliminary unadjusted results for 2022 and the comparable results for 2021 are presented on a pro forma basis assuming GeneDx and Sema4 were combined for the entirety of 2021 and 2022 and exclude the revenues and costs from the exited reproductive health and somatic tumor testing businesses. Actual results may differ materially from those disclosed in this presentation and will include the results of the legacy GeneDx business only from the date of Sema4 Holdings Corp.’s acquisition of GeneDx on April 29, 2022, the purchase accounting associated with the acquisition of GeneDx, and will also include the financial impacts of exited Sema4 business activities for the full year. 2. Adjusted gross margin is a non-GAAP financial measure. GeneDx has not provided a reconciliation of jts preliminary, unaudited Adjusted Gross Margin to the most directly comparable GAAP measure because certain items excluded from GAAP cannot be reasonably calculated or predicted at this time. Accordingly, a reconciliation is not available without unreasonable effort.

v Revenues between $205-220 million for full year 2023 Expanded adjusted gross margin profile 2023 and beyond Inclusive of servicing obligations of the exited business activities, total cash burn in 2023 between $130-145 million, a >50% reduction year-over-year Use of net $95-110 million in cash in 2023 for continuing operations Turn to profitability in 2025 2023 Guidance

Driving growth and expanding markets

Quickly growing into a significant opportunity Conservatively, our diagnostic testing total addressable market is ~$30 billion1 Newborn Screening: $10B Eventual inclusion of genetics in screening programs Underdeveloped Solution for Sema4 Adults: $16B Rapidly expanding into cardio and neuro to replace status quo panels and individual gene tests Current Gap for Sema4 Rapidly growing patient opportunity and substantial cost savings via early screening 1 Company Estimates supported by DefinitiveHC diagnosis data Rare Disease & Pediatrics: $3B

Starting with rare disease and pediatrics •>7000 individual diseases affecting 10% of the total population • • 400 million people worldwide • • 50% of people affected by rare diseases are children • • 700 medicines in development – same as oncology with regulatory pathway via Orphan Drug Act

NICU Patient Test ordered Today’s strategy expedites diagnoses, saves money per patient with rapid exome and genome sequencing Starting with the most vulnerable patients Rapid results ✓ Patient retention ✓ Patients connected ✓ Healthier outcomes ✓ $6,8002 savings per diagnosis when tested at first tertiary presentation for Pediatric Delay Disorder (outpatient) Faster answer à Better outcomes à Lower Costs $30,0001 per case average savings in the NICU from reduced length of stay, unnecessary care (Inpatient) 1ScienceDaily. (2017, October 19). Rapid whole-genome sequencing of neonatal ICU patients is useful and cost-effective. ScienceDaily. 2Tan TY, Dillon OJ, Stark Z, et al. Diagnostic Impact and Cost-effectiveness of Whole-Exome Sequencing for Ambulant Children With Suspected Monogenic Conditions. JAMA Pediatrics. 2017;171(9):855. doi:10.1001/jamapediatrics.2017.1755
+500 With a rapidly growing and loyal customer base Geneticist Neonatologist Pediatrician Neurologist Cardiologist Hospital Lab Director • Leveraging decades of earned trust amongst expert geneticists to expand into incremental call points and use cases • •94% of pediatric specialists in the U.S. who order exome testing have ordered from GeneDx in the past 12 months1. 1Company estimates, Definitive Healthcare

Emerging guidelines and medical policies underpin rapid growth ACMG Practice Guideline published July 2021: “Strong recommendation based on the available evidence to support the use of ES/GS as either a first- (or second-) line test in patients …. ES/ GS demonstrates clinical utility for the patients and their families with limited evidence for negative outcomes and the ever increasing emerging evidence of therapeutic benefit.” ~70% of commercial payers have coverage for exome / WGS, when criteria are met and an ever growing number of commercial payers and Medicaid programs are adopting favorable coverage1. In January 2023, UnitedHealthcare® issues Medical Policy Update Bulletin which provides Exome/Whole Genome Sequencing coverage across their Commercial Plans, effective March 1, 2023 NSGC Guideline recommended October 2022: “Recommending Exome Sequencing as a First- Tier Genetic Test for Unexplained Epilepsies” 1Company estimates, Definitive Healthcare American Epilepsy Society recommends…Exome or genome sequencing are favored for most scenarios, as they are more likely to provide a diagnosis.

Transforming the standard of care October 27, 2022 Sema4 | GeneDx Announces Results from Phase 1 of Seq First Study, Demonstrating Broad Utility of Rapid Whole Genome Sequencing for Critically Ill Newborns October 5, 2022 Sema4 | GeneDx To Provide Whole Genome Sequencing and Interpretation Services for Landmark Genomic Newborn Screening Study October 26, 2022 Sema4 | GeneDx collaborates on new research demonstrating genome and exome sequencing deliver more diagnostic certainty than multi-gene panels July 2021 GeneDx one of only two labs selected to partner with UnitedHealthcare® in a NICU rapid exome sequencing program
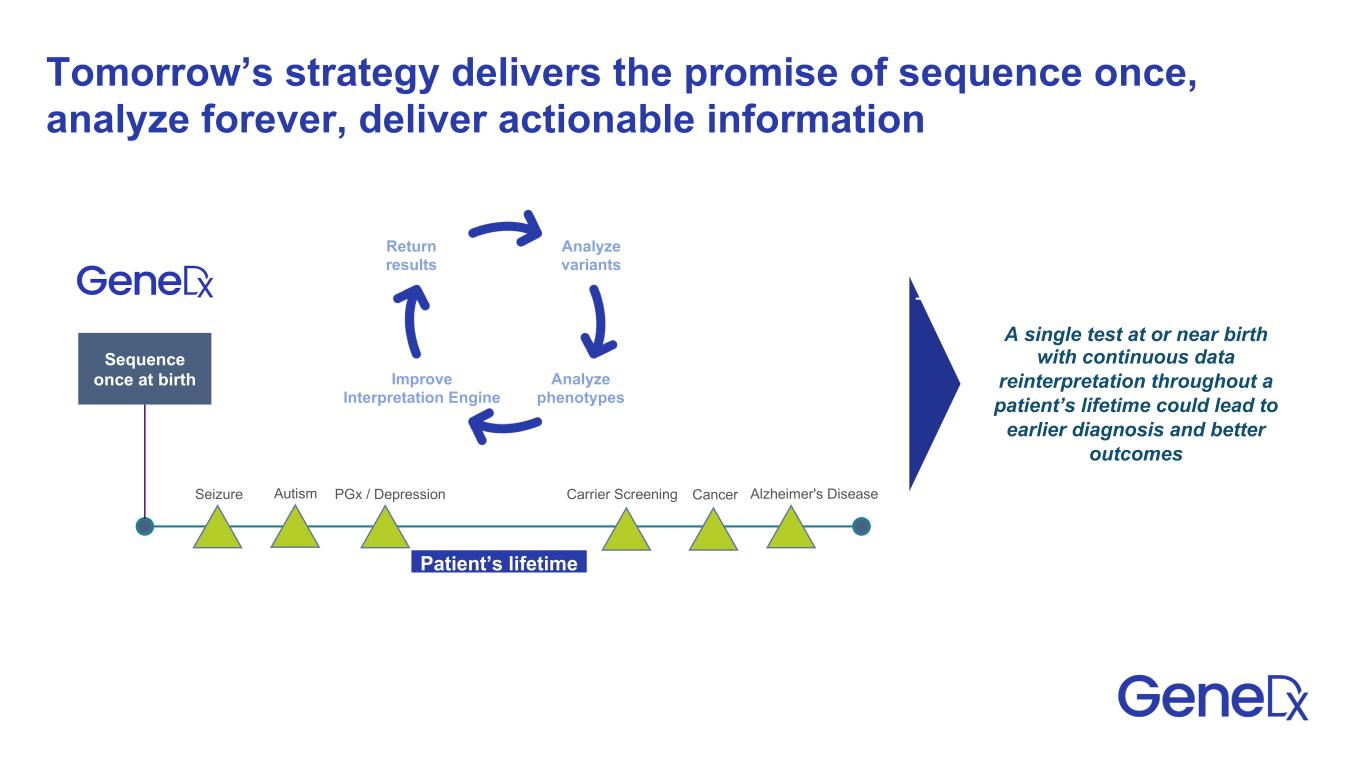
Tomorrow’s strategy delivers the promise of sequence once, analyze forever, deliver actionable information Patient’s lifetime A single test at or near birth with continuous data reinterpretation throughout a patient’s lifetime could lead to earlier diagnosis and better outcomes Sequence once at birth Seizure Autism PGx / Depression Carrier Screening Cancer Alzheimer's Disease Return results Analyze variants Analyze phenotypes Improve Interpretation Engine

Miss Less. Discover More. Our unparalleled interpretation platform.

Delivering fewer uncertain findings, critical for expanding utilization Return results Analyze variants Analyze phenotypes Improve Interpretation Engine • Fewer uncertain findings lead to improved clarity for clinicians and patients • More expertly curated disease-causing variants vs. public data sets • Definitive diagnosis in more cases Public curation tool* BENIGN UNCERTAIN Review likely required GeneDx proprietary software Fewer complex variants for review BENIGN UNCERTAIN Review likely required Review not needed *ClassifyCNV Review not needed

The snowball effect of accumulated GeneDx data identifies more pathogenic findings that others miss Lab A GeneDx Panels leave patients behind. Exome and genome do not.

Untapped partners: biopharma In 2020 alone, more than half (55%) of novel new drug and biological approvals were orphan drugs for rare diseases.

FIND 0 - 3 year focus UNDERSTAND 1 - 5 year focus PLATFORM Long Term M ar ke t O pp or tu ni ty FIND Recurring services model for identifying Rare Disease patients for clinical trial recruitment or delivery of targeted Rx UNDERSTAND Reports w/analytics leveraging clinicogenomic data in Rare Disease and Oncology within Centrellis to support R&D for targeted therapies PLATFORM Combined SaaS offering: Therapeutic Area agnostic access to data, patients and insights for RWE/RWD to support end- to-end drug discovery pipeline Data strategy leverages Centrellis as a solutions provider Biopharma research offerings unlock upside as platform expands

Experienced management team with decades of leadership in genetics, data science, healthcare Gustavo Stolovitzky Chief Science Officer Katherine Stueland Chief Executive Officer Jennifer Brendel Chief Commercial Officer Matthew Davis Chief Technology & Product Officer Kevin Feeley Chief Financial Officer Kareem Saad Chief Transformation Officer Karen White Chief People Officer

One test. There's no one better.
